"Wellness for All"
- Call Us: +91 44 2472 2244
- Mail Us: md@gsbpl.com
GreenSignal Bio Pharma Private Limited
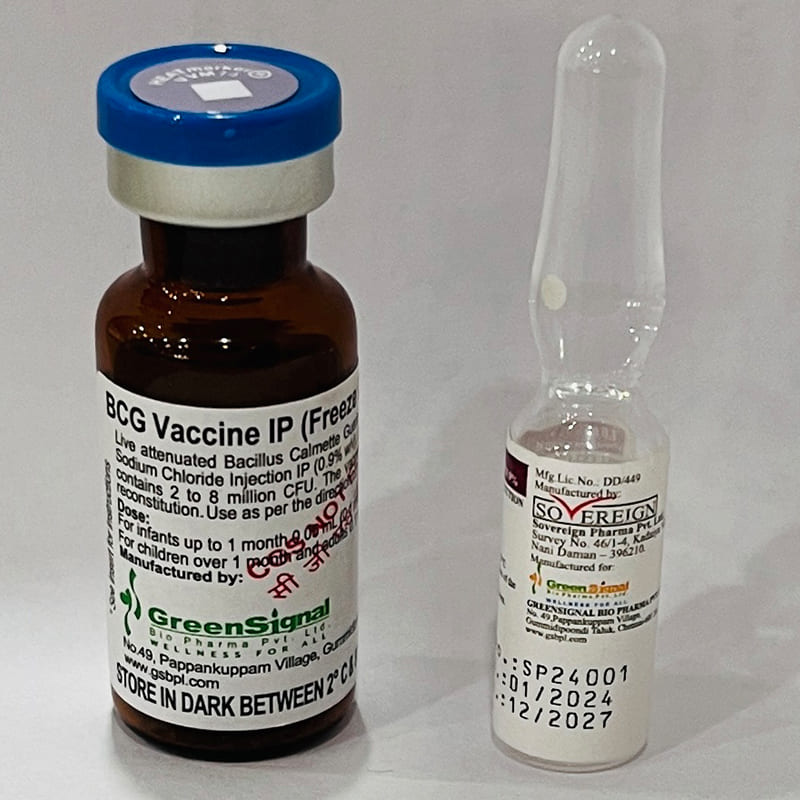
BCG Vaccine is freeze-dried live attenuated Bacillus Calmette - Guerin derived from strain of Mycobacterium for the prevention of Tuberculosis
The vaccine has to be reconstituted with 1.0 ml of sodium chloride injection (0.9% w/v).
After reconstitution, a dose of 0.05ml is equivalent to 1 to 4 × 10 5 CFU and a dose of 0.1 ml is equivalent to 2 to 8 × 10 5 CFU.
The Vaccine has to be administered intradermally.
BCG vaccine as a well-established and effective defense against tuberculosis, a serious infectious disease. Protecting Children from Tuberculosis (TB)
GreenSignal Bio Pharma Private Limited
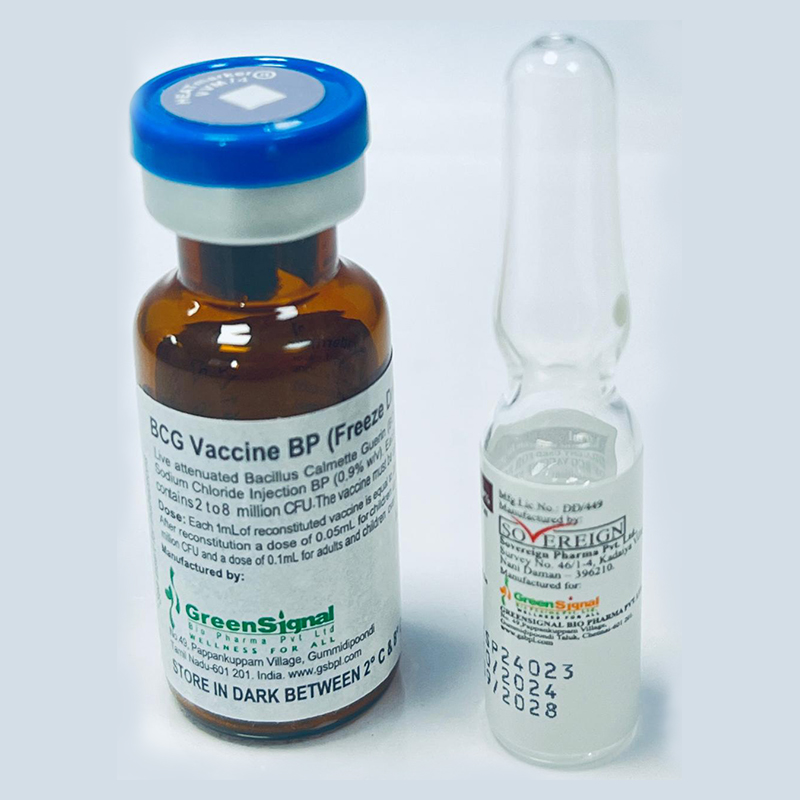
The vaccine is a lyophilised active component and has to be reconstituted with 0.9% Sodium Chloride diluent before use.
Each 1ml of reconstituted vaccine is equal to 20 doses (0.1 to 0.4 million CFU/dose) for children up to 1 year/ Each 1ml of reconstituted vaccine is equal to 10 doses (0.2 to 0.8 million CFU/dose) for children over 1 year.
Number of Doses: 20
Route of Administration: Intradermal
Shelf Life: 24 months
Storage Temperature: 2 °C to 8 °C. Protected from direct light
Vaccine Vial Monitor: Type 14
Multidose Vial Policy:
The opened vials of this vaccine should be discarded 6 hours after opening or at the end of the immunization session, whichever comes first.
Vaccine Preservative: None
Cold Chain Volume
VACCINE: 1.43 (0.1 mL dose) / 0.71 (0.05 mL dose).
DILUENT: 0.82 (0.1mL dose) / 0.41 (0.05 mL dose) cm3/dose (in secondary packaging)
GreenSignal Bio Pharma Private Limited
BCG ONCO for Immunotherapy is a live, attenuated freeze-dried preparation
Bacterial Suspension: 40mg
Monosodium glutamate: 5.0 % w/v
Administration: Intra-vesical Instillation.
The Manufactured BCG Vaccine and BCG Vaccine for Immunotherapy (ONCO) are stored in cold room ( 2 to 8° C) and the packaging procedure is as per regulatory requirements.
Bacillus Calmette-Guerin (BCG) treatment is a type of intravesical (in bladder) immunotherapy. This lyophilized drug is made from a strain of Mycobacterium bovis. It is one of the most effective treatments for high-risk non-muscle-invasive bladder cancer.

The SmPC is a legal document approved as part of the marketing authorisation of each medicine.
The SmPC is the basis of information for healthcare professionals on how to use the medicine.
Detailed Description of BCG Vaccine IP Detailed Description of BCG Vaccine BP